Utilization Overview
The iCROWN repository collects, manages, and stores specimens such as blood, swabs, saliva, etc. collected from patients, as well as medical information, for severe acute respiratory infections (including Coronavirus disease 2019), mpox, pediatric hepatitis of unknown etiology, and specimens collected through genomic surveillance at the time of entry into Japan. In addition, collected specimens are subjected to genome analysis, and the results are also stored and managed in the repository.
Those wishing to utilize these specimens and data must first pass a screening process.
The repository’s provision framework is based on the concept of a trustworthy custodian. While leveraging the benefits of traditional collaborative research agreements and provision (based on MTAs or DTAs), it proposes a new approach to external biobank distribution that differs from both. iCROWN users will retain the results and authorship of their utilization of the repository’s specimens and data. However, they are required to report on the utilization status and cooperate with iCROWN’s investigations.
For details, please contact the inquiry desk (iCROWN Utilization Desk).
Basic Utilization Policy
- Utilization is available to researchers affiliated with research institutions and companies in Japan.
- Research results obtained utilizing specimens and data from the iCROWN repository belong to the user.
- When publishing research results, users are required to acknowledge the utilization of data from the iCROWN repository and include a mention in the methods section.
- Usage fees are free for both corporate and academic organizations for research that is consistent with the iCROWN repository’s purpose. However, costs necessary for the provision of specimens and data, such as shipping fees, will be the responsibility of the user.
Utilization Process
If you wish to utilize the repository, please follow the steps outlined below.
Depending on the application status, a waiting period may be required for step 5) Research consultation.
Utilization Process (click to display flowchart)
- 1) Preliminary account registration
- If you already have an account for the iCROWN Repository Portal, you may skip steps 1) to 3) and proceed directly to step 4).
If you do not have an account, please click [here] for preliminary account registration.
- 2) Account issuance application
- After completing the preliminary account registration, submit an account issuance application. You may also apply for accounts for your co-researchers at the same time.
- 3) Complete e-Learning
- Once you have been issued an account, you will be required to take three e-learning courses (research ethics, pathogen training, and ethics guidelines).
- 4) Browse the showcase
- After completing the e-learning and having your status approved, you can search for specimens and data collected in the iCROWN repository. Please use this information as a reference when considering your application. Log in to the Repository Portal, and select “launch showcase” from the utilization dropdown menu.
- 5) Research consultation
- Contact the iCROWN Utilization Desk via email. We will provide you with an overview of the specimens and data within the iCROWN repository and discuss with you what will be used in your research.
- 6) Utilization application
- If the consultation confirms that you can proceed with utilization, we will send you the necessary forms by email. Please fill these out. Once you submit the necessary application documents, we will review them. After confirming that all necessary information has been properly completed, the utilization subcommittee will deliberate on the utilization.
- 7) Receive specimens and data
- Upon approval by the utilization subcommittee, a Material Transfer Agreement (MTA) will be concluded.The specimens will then be shipped to you from the Japan Institute for Health Security. Data will be provided to you by the analysis laboratory within the Japan Institute for Health Security.
- 8) Report research outcomes
- Please note that a utilization report must be submitted each fiscal year. Additionally, any research achievements, such as published papers, must also be reported.
Specimen and Data Submission Process (remaining specimens from existing research, etc.)
It is possible to cooperate with iCROWN by offering to provide specimens or data that were collected through other research or clinical trials separate from iCROWN. We will consider accepting your existing specimens and data into the iCROWN repository on a case-by-case basis. Please contact the inquiry desk (iCROWN Utilization Desk) for inquiries.
Specimen and Data Submission Process (click to display flowchart)
- 1) Preliminary consultation
- If you wish to provide specimens or data to the iCROWN repository, please contact the iCROWN Utilization Desk via email. We will provide you with further information.
- 2) Preliminary account registration
- If you already have an account for the Repository Portal, you may skip steps 2) to 4) and proceed to step 5).
If you do not have an account, please click [here] for preliminary account registration.
- 3) Account issuance application
- After completing the preliminary account registration, submit an account issuance application. You may also apply for accounts for your co-researchers at the same time.
- 4) Complete e-Learning
- Those issued an account will be required to take three e-learning courses (research ethics, pathogen training, and ethics guidelines).
- 5) Download required forms
- Various required forms are available to be downloaded from the Repository Portal.
- 6) Specimens/data submission procedure
- Please submit ethics review materials and all other necessary documents to iCROWN. Following approval by the utilization subcommittee and the signing of the contract, specimens and data may be submitted. If requested, we can provide you with the human and pathogen genome sequence data obtained from the specimens you provied.
Terms, MTA, and Related Documents
Please refer to Public Documents > repository related.
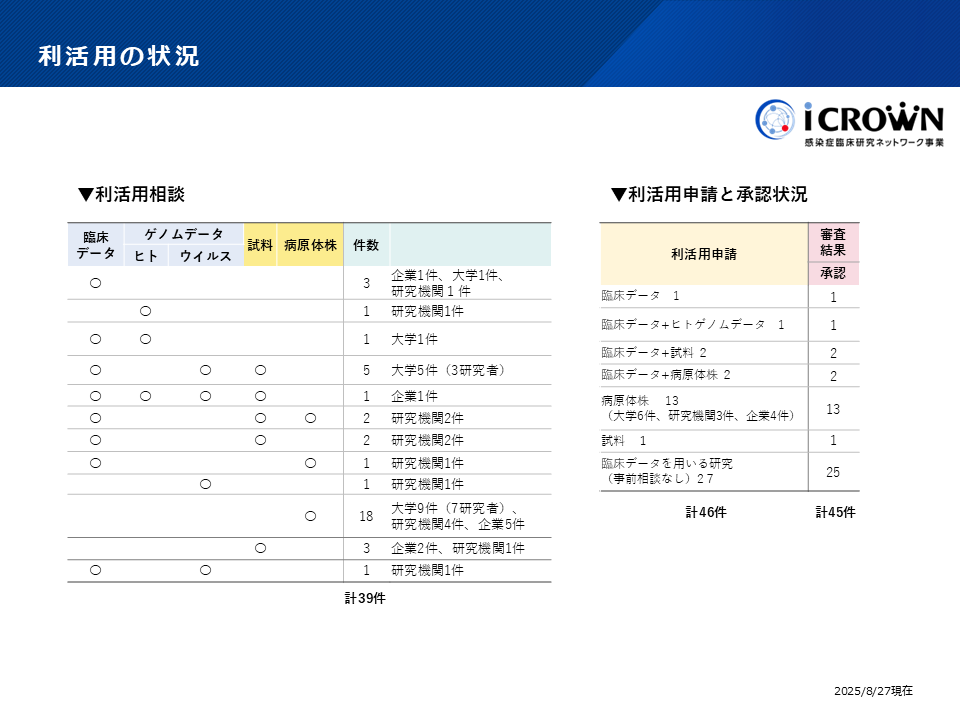
Utilization Status
Research Utilizing the Repository
For more information on research utilizing the repository, please click here.
